EU CTR: How to write a good Lay Summary of clinical study results

Dr. rer.nat. Ramona Schütz | GKM Gesellschaft für Therapieforschung mbH
What are Lay Summaries and what is the aim?
Lay summaries are a display of clinical study results intended for study participants and the general public. The aim of a lay summary is not only to enhance understanding of complex medical information, but also to increase transparency in clinical research.1 This effort is supported by an increasing interest of study participants in study results and call for greater transparency.2 Providing a lay summary for all clinical trials – irrespective of the clinical trial phase and outcomes – is for the first time a mandatory requirement by the European Union Clinical Trials Regulation (EU CTR) 536/20143 and came fully into force by end of January 2022. Sponsors must upload a lay summary that will then be publicly accessible through the Clinical Trials Information System (CTIS). After a transition period of 3 years, the submission of lay summary to the CTIS will be mandatory from 31 January 2025 onwards for all clinical trials, both ongoing and new ones (Picture 1).4
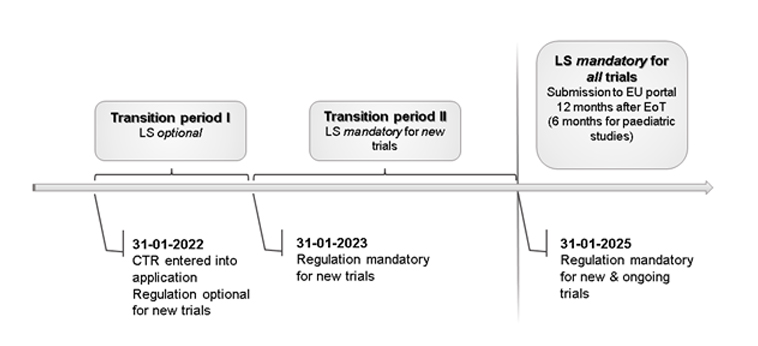
Picture 1: Lay Summary (LS) submission during transition period: LS submission optional for trials until 31-01-2023, from then until 31-01-2025 LS submission mandatory for new trials; from 31-01-2025 LS submission mandatory for all clinical trials, both new and ongoing.
What are the requirements?
Article 37 of the EU CTR 536/2014 highlights regulatory requirements. Annex V of the regulation defines 10 items to be essential content that your lay summary should cover:5
As the target audience of the summaries are laypersons with limited health literacy or scientific expertise the summaries must be provided in plain language, which is easily understandable. The lay summary authors must describe study designs and endpoints in a lay-friendly manner avoiding technical terms. The EU CTR guidance recommends the use of literacy proficiency level of 2–3 on the International Adult Literacy Survey Scale.6 The visualization of content, e.g. the use of infographics for better understanding is essential. For the paediatric audience sponsors can consider a graphical presentation in the form of an illustration or even a cartoon.1,6,7
If the trial was conducted in several countries, a translation of the lay summary in all local languages is required. The content must be strictly non-promotional in all aspects, e.g. presentation of results must be objective and non-selective; use of substance names is preferred over tradenames. To ensure readability of the lay summary, sponsors are highly encouraged to involve laypersons, in particular patients or patient organisations, early on in the development and the review process.6 Publishing of lay summary via CTIS is obligatory, but different ways of dissemination e.g. sharing on a website, direct mailing to study participants through third parties or dissemination through the investigative site can be considered.
What are the benefits?
„Embrace the value of helping the public understand science.”8
Lay summaries are not only a way to meet the desire for transparency and the increasing public interest in clinical research, but also aid to “demystify the clinical study process”.1 Returning the results to study participants is not just meeting a requirement, but a unique opportunity to acknowledge the patient’s participation and the value of their contribution to medical research and public health. This in turn can promote trust and enhance public engagement as well as raise the awareness of the relevance of medical research. Not least, the COVID-19 pandemic revealed the importance of public health communication and the early involvement of the public in health-related issues. A lack of transparency and limited information sharing on the other hand can breed mistrust and leave space for misinformation and myths.9 Thus, lay summaries bear the potential to make clinical study results accessible and understandable to the broad lay public and maintain public trust in science and medical research.
Current challenges and best practices
Lay-friendly language & graphics
The creation of a lay summary might be time-consuming and costly. The achievement of appropriate lay-level readability and sound graphical presentation might require special writing and design skills, specifically when creating lay summary for a paediatric audience. To achieve optimal results it is useful to engage experienced medical writers and graphic designers. Furthermore, the European Expert Group provides recommendations including suitable descriptions in plain language for common endpoints used in clinical trials.6
Presentation of safety information
Another challenge is the presentation of safety information. Generally, a description of the most frequent adverse events related to the investigational product (drug-related adverse events) is sufficient. However, readers may confuse drug-related adverse events captured at trial level with side effects presented in the package leaflet.1 Medical Dictionary for Regulatory Activities (MedDRA) preferred terms are usually difficult to understand by laypersons. A simple but accurate description as well as a concise glossary is useful to overcome this challenge.
Non-promotional tone
Sponsors need to keep in mind that the lay summary must be strictly non-promotional. Anything that could lead to possible consideration as promotional material must be avoided. This includes not only the presentation of results, but also the visual appearance and way of dissemination. Thus it is strongly recommended to avoid tradenames, create a balanced and objective presentation (i.e. do not highlight positive outcomes while minimizing negative results), and avoid absolute claims (e.g. “the results clearly proved”) as much as possible. If lay summaries are published on a website, only non-promotional content should be hosted.1
Review process & translation
When translating the lay summary, translators need to ensure that the translation is accurate and that native readers consider the language adequate. Additionally, the translation bears the risk of creating content differences between the master version and the translated summaries. Therefore, a back translation process is encouraged.
Lastly, as the early involvement of laypersons, in particular patients, in the development and review process is highly encouraged, sponsors should identify and network with patient associations, representatives or advocacy groups. Once relationship is established, this cooperation holds the potential benefit of directly engaging with key stakeholders.
References
- Barnes A, Patrick S. Lay Summaries of Clinical Study Results: An Overview. Pharmaceut Med. 2019;33(4):261-268.
https://doi.org/10.1007/s40290-019-00285-0
- Center for Information & Study on Clinical Research Participation. Perceptions and Insights Study.
https://www.ciscrp.org/wp-content/uploads/2021/11/2021-PI-ALL.pdf - THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC (Text with EEA relevance) 2014. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32014R0536
- European Medicines Agency. Clinical Trails Regulation.
https://www.ema.europa.eu/en/human-regulatory/research-development/clinical-trials/clinical-trials-regulation - THE EUROPEAN PARLIAMENT AND THE COUNCIL OF THE EUROPEAN UNION. Annex V of Regulation (EU) No 536/2014: CONTENT OF THE SUMMARY OF THE RESULTS OF THE CLINICAL TRIAL FOR LAYPERSONS. 2014.
- European Medicines Agency. Summaries of Clinical Trial Results for Laypersons: Recommendations of the expert group on clinical trials for the implementation of Regulation (EU) No 536/2014 on clinical trials on medicinal products for human use – Version 2. 2018.
- Regulatory Affairs Professional Society, ed. Regulatory Writing: An Overview: Chapter 29: Lay Summaries of Clinical Study Results. 2nd Edition; 2020.
- Monica Duke. How to write a good lay summary: A Digital Curation Centre (DCC) ‘working level’ guide – in collaboration with the Patients Participate! project.
https://www.dcc.ac.uk/sites/default/files/documents/publications/HowToLaySummariesDec2012.pdf - Porat T, Nyrup R, Calvo RA, Paudyal P, Ford E. Public Health and Risk Communication During COVID-19-Enhancing Psychological Needs to Promote Sustainable Behavior Change. Front Public Health. 2020;8:573397.
https://doi.org/10.3389/fpubh.2020.573397
Picture: @Blue Planet Studio/AdobeStock.com









